SAFETY DATA SHEET
Silver Nitrate
1. IDENTIFICATION
Product Identifiers
Product Name:
Silver Nitrate, ACS/ISO Grade
Other Names:
Silver Nitrate AR, Silver Nitrate LR
Product Number(s):
C139, C1391, C1395
CAS Number:
7761-88-8
Recommended use of the chemical and restriction on use
Laboratory use. Photographic film, catalyst for ethylene oxide, indelible inks, silver plating, silvering mirrors, silver salts, germicide (as a wall spray), hair dyeing, analytical chemistry, antiseptic, purification of drinking water, fused form to cauterise wounds and laboratory reagent.
Company Details
ProSciTech Pty Ltd
11 Carlton Street
KIRWAN QLD 4817
Australia(07) 4773 9444www.proscitech.com
11 Carlton Street
KIRWAN QLD 4817
Australia(07) 4773 9444www.proscitech.com
Emergency Contact Details
ProSciTech Pty Ltd
11 Carlton Street
KIRWAN QLD 4817
Australia(07) 4773 9444www.proscitech.com
11 Carlton Street
KIRWAN QLD 4817
Australia(07) 4773 9444www.proscitech.com
2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Oxidising Solids: Category 2 - H272 May intensify fire; oxidiser
Acute Toxic: Category 2 - H300 Fatal if swallowed Eye Damage/Irritation: Category 1 - H318 Causes serious eye damage
Skin Corrosion/Irritation: Category 1B - H314 Causes severe skin burns and eye damage
Acute Toxic: Category 2 - H300 Fatal if swallowed Eye Damage/Irritation: Category 1 - H318 Causes serious eye damage
Skin Corrosion/Irritation: Category 1B - H314 Causes severe skin burns and eye damage
Label Elements
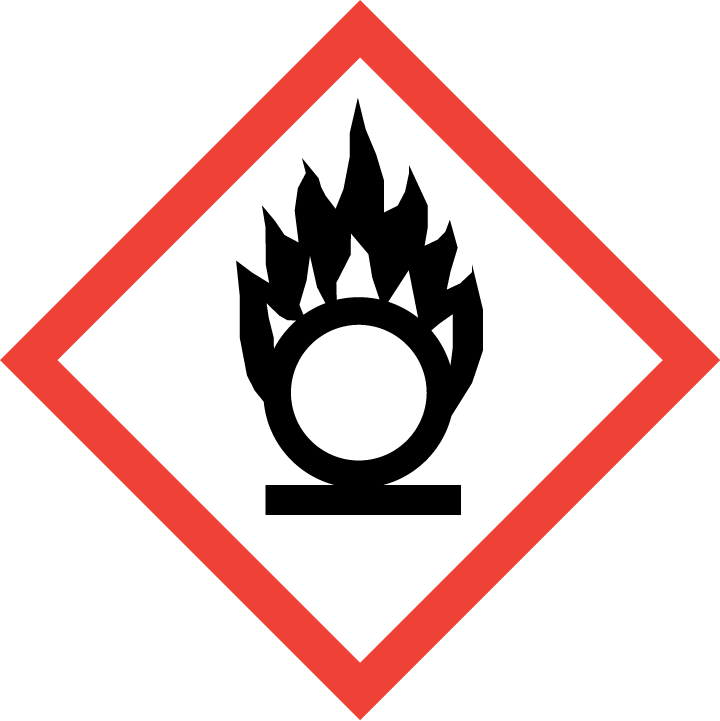
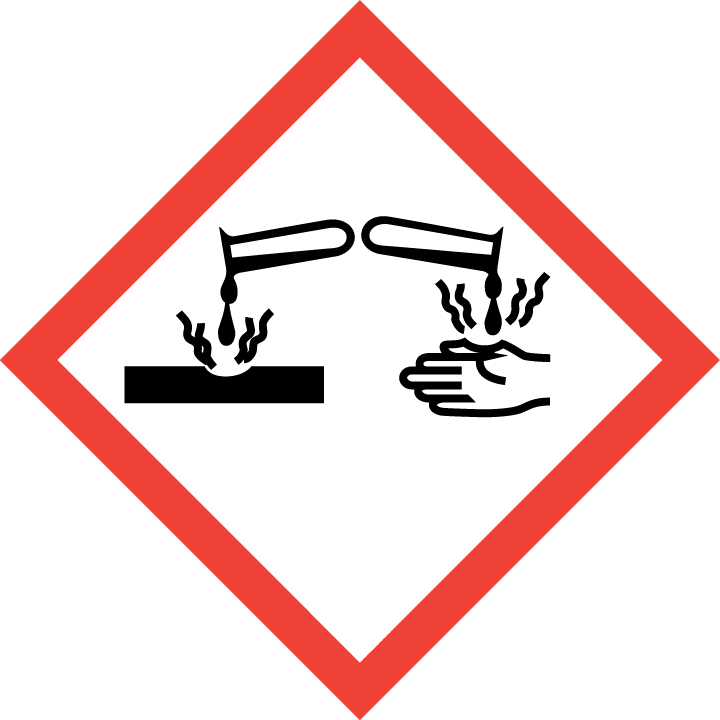

Signal Words
Danger
Hazard Statement(s)
H272 May intensify fire; oxidiser.H304: May be fatal if swallowed and enters airwaysH314 Causes severe skin burns and eye damage.
Precautionary Statement(s)
P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
P220 Keep/Store away from clothing/.../combustible materials.
P221 Take any precaution to avoid mixing with combustibles ...
P260 Do not breathe dust/fume/gas/mist/vapours/spray.
P264 Wash thoroughly after handlingP301+P310: If swallowed: Immediately call a poison center/doctorP301+P330+P331 - If swallowed: Rinse mouth. Do not induce vomitingP303+P361+P353: If on skin (or hair) Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340: If Inhaled. remove person to fresh air and keep comfortable for breathingP305+P351+P338 If in eyes: Rince cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P321: Specific treatment (see this label)P363: Wash contaminated clothing before reuseP405 Store locked up.P501: Dispose of contents/container in accordance with local regional/national/international regulations.P273 Avoid release to the environment.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
P220 Keep/Store away from clothing/.../combustible materials.
P221 Take any precaution to avoid mixing with combustibles ...
P260 Do not breathe dust/fume/gas/mist/vapours/spray.
P264 Wash thoroughly after handlingP301+P310: If swallowed: Immediately call a poison center/doctorP301+P330+P331 - If swallowed: Rinse mouth. Do not induce vomitingP303+P361+P353: If on skin (or hair) Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340: If Inhaled. remove person to fresh air and keep comfortable for breathingP305+P351+P338 If in eyes: Rince cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P321: Specific treatment (see this label)P363: Wash contaminated clothing before reuseP405 Store locked up.P501: Dispose of contents/container in accordance with local regional/national/international regulations.P273 Avoid release to the environment.
P280 Wear protective gloves/protective clothing/eye protection/face protection.
Primary route(s) of entry
Not available.
Human Health
Inhalation:
P304+P340 IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
P310 Immediately call a POISON CENTRE or doctor/physician.
P310 Immediately call a POISON CENTRE or doctor/physician.
Ingestion:
P301+P330+P331 IF SWALLOWED: rinse mouth. Do NOT induce vomiting. Drink copious amounts of water and rovide freash air. P310 Immediately call a POISON CENTRE or doctor/physician
Eyes:
P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Consult medical advise
Skin:
P303+P361+P353 IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
P363 Wash contaminated clothing before reuse.
P363 Wash contaminated clothing before reuse.
Environment
Inform respective authorities in case of seepage into water course or sewage systemDO NOT allow to enter sewage system or ground water
3. COMPOSITION/INFORMATION ON INGREDIENTS
Name
CAS No.
Content (w/w)
Classification
Silver nitrate
7761-88-8
100%
-
4. FIRST AID MEASURES
Ingestion
Rinse mouth thoroughly with water immediately, repeat until all traces of product have been removed.
DO NOT INDUCE VOMITING. Seek immediate medical advice.
DO NOT INDUCE VOMITING. Seek immediate medical advice.
Inhalation
If inhaled, remove from contaminated area to fresh air immediately. Apply artificial respiration if not breathing. If breathing is difficult, give oxygen. Immediately obtain medical aid if cough or other symptoms appear.
Skin Contact
Wash affected area thoroughly with copious amounts of running water. Remove contaminated clothing and wash before reuse. If symptoms develop seek medical attention.
Eye Contact
Immediately irrigate with copious quantity of water for at least 15 minutes. Eyelids to be held open.
Seek medical attention.
Seek medical attention.
Other Information
No further relevant information available.
5. FIREFIGHTING MEASURES
Suitable extinguishing equipment
USE FLOODING QUANTITIES OF WATER. Use dry chemicals, CO₂ powder or water spray. If safe to do so, move undamaged containers from fire area. Do not move cargo if cargo has been exposed to heat.
HAZCHEM
1Y
Special protective equipment and precautions for fire fighters
Will accelerate burning when involved in a fire. May explode from heating, shock, friction or contamination. May react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, clothing, etc). Fire may produce irritating, poisonous, and/or corrosive gases. Containers may explode when heated. Runoff may create fire or explosion hazard.
Decomposition Temp. 440°C
6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Eliminate all ignition sources (no smoking, flares, sparks or flame) within at least 15m. Do NOT touch or walk through this product. Stop leak if safe to do so. Prevent entry into waterways, drains, confined areas. Prevent dust cloud. Use clean non-sparking tools to collect material and place it into loosely-covered plastic containers for later disposal.
SEEK EXPERT ADVICE ON HANDLING AND DISPOSAL
SEEK EXPERT ADVICE ON HANDLING AND DISPOSAL
Environmental precautions
Not available
Methods and materials for containment and clean up
Sweep up (avoid generating dust) and using clean non-sparking tools transfer to a clean, suitable, clearly labelled container for disposal in accordance with local regulations.
Most organisations using silver compounds collect all silver residues for subsequent recovery. Any solids spilt may be swept up for eventual recovery or disposal. Solutions could be washed to drain with a large volume of water, or alternatively treated with a salt solution and the resulting silver chloride collected for subsequent recovery.
Most organisations using silver compounds collect all silver residues for subsequent recovery. Any solids spilt may be swept up for eventual recovery or disposal. Solutions could be washed to drain with a large volume of water, or alternatively treated with a salt solution and the resulting silver chloride collected for subsequent recovery.
7. HANDLING AND STORAGE
Precautions for safe handling
Avoid ingestion and inhalation of vapour or dust. Avoid contact with eyes, skin, clothing and other combustible materials. Avoid prolonged or repeated exposure. If ingested, seek medical advice immediately and show the container or the label. Minimize dust generation and accumulation. Ensure good ventilation at the workplace. Use with adequate ventilation. In case of insufficient ventilation, wear suitable respiratory equipment. This substance is an oxidiser and its heat of reaction with reducing agents or combustibles may cause ignition. Heat, shock, friction, or contact with other materials may cause fire or explosion. Keep away from heat and all sources of ignition - No smoking. Keep container dry. Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Discard contaminated shoes.
Conditions for safe storage
Unsuitable Materials: Wooden, metal, cardboard or paper.
8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Exposure Standards
Material
TWA ppm
TWA mg/m3
STEL ppm
STEL mg/m3
Silver nitrate
-
0.01
-
-
Engineering controls
Maintain eyewash fountain and safety shower in work area.
In industrial situations maintain the concentrations values below the TWA. This may be achieved by process modification, use of local exhaust ventilation, capturing substances at the source, or other methods.
In industrial situations maintain the concentrations values below the TWA. This may be achieved by process modification, use of local exhaust ventilation, capturing substances at the source, or other methods.
Personal protective equipment
Eye and face protection
The use of a face shield, chemical goggles or safety glasses with side shield protection as appropriate.
Must comply with Australian Standards AS 1337 and be selected and used in accordance with AS 1336.
Must comply with Australian Standards AS 1337 and be selected and used in accordance with AS 1336.
Skin protection
Hand protection should comply with AS 2161, Occupational protective gloves - Selection, use and maintenance.
Recommendation: Excellent: NR latex, vinyl and neoprene. Good: Nitrile rubber gloves
Recommendation: Excellent: NR latex, vinyl and neoprene. Good: Nitrile rubber gloves
Body protection
Final choice of personal protective equipment will depend on individual circumstances and/or according to risk assessments undertaken.
Flame retardant protective clothing. Clean clothing or protective clothing should be worn, preferably with an apron. Clothing for protection against chemicals should comply with AS 3765 Clothing for Protection Against Hazardous Chemicals.
Flame retardant protective clothing. Clean clothing or protective clothing should be worn, preferably with an apron. Clothing for protection against chemicals should comply with AS 3765 Clothing for Protection Against Hazardous Chemicals.
Respiratory protection
Where ventilation is not adequate, respiratory protection may be required. Avoid breathing dust, vapours or mists. Respiratory protection should comply with AS 1716 - Respiratory Protective Devices and be selected in accordance with AS 1715 - Selection, Use and Maintenance of Respiratory Protective Devices. Filter capacity and respirator type depends on exposure levels. In event of emergency or planned entry into unknown concentrations a positive pressure, full-face piece SCBA should be used. If respiratory protection is required, institute a complete respiratory protection program including selection, fit testing, training, maintenance and inspection.
9. PHYSICAL AND CHEMICAL PROPERTIES
General information
Appearance
Solid Colourless, transparent, tabular, rhombic crystals or white crystalline powder, becoming gray or grayish-black on exposure to light in the presence of organic matter.
Odour
Odourless
pH
Aqueous and alcoholic soln are neutral to litmus; pH 5.4 - 6.4 (100 g/l H2O).
Vapour Pressure
Not available
Density
5.8
Boiling Point
433 °C
Melting Point
212 °C
Solubility
Very soluble, 1220 g/l at 0 °C
Specific Gravity of Density
4.352
Flash Point
Not available
Flammable (Explosive) Limits
Not combustible but assists combustion of other substances
Ignition Temperature
Many reactions may cause explosion.
Reacts with ammonia to form compounds that are sensitive to mechanical shock.
Silver nitrate mixed with dry powdered magnesium may ignite explosively on contact with a drop of water.
An explosive fulminate may be formed if silver nitrate is mixed with alcohols
Reacts with ammonia to form compounds that are sensitive to mechanical shock.
Silver nitrate mixed with dry powdered magnesium may ignite explosively on contact with a drop of water.
An explosive fulminate may be formed if silver nitrate is mixed with alcohols
Formula
AgNO3
10. STABILITY AND REACTIVITY
Reactivity
No further relevant information available
Chemical stability
Stable at room temperature in closed containers under normal storage and handling conditions. Light sensitive. Darkens on exposure to light.
Possibility of hazardous reactions
Reacts with acetylene in presence of ammonia to form silver acetylide, a sensitive powerful detonator when dry. In the absence of ammonia, or when calcium acetylide is added to a silver nitrate soln, explosive double salts of silver acetylide and silver nitrate are produced. Mercurous acetylide precipitates silver acetylide from aqueous nitrate. Reaction with chlorosulfonic acid is violent with nitrosulfonic acid being formed. Reduced by hydrogen sulfide in the dark. Easily reduced to metallic silver by ferrous salts, arsenites, hypophosphites, tartrates, sugars, tannins, volatile oils. Dry powdered magnesium and silver nitrate may ignite explosively on contact with a drop of water. Reaction with ammonium hydroxide, sodium hydroxide and stirring may be explosive. Reaction with phosphorus, or sulphur, and shock may be violently explosive. Reaction with charcoal and shock may result in ignition. Highly sensitive explosive is formed when calcium carbide is added to silver nitrate solution. Reaction with alcohols may form an explosive fulminate. When purified phosphine was passed rapidly into a concentrated solution of silver nitrate an explosion occurred.
Conditions to avoid
Heat, flame, sources of ignition, light, contamination and incompatible materials.
Incompatible materials
Reducing agents, combustible materials, organic materials, easily oxidised materials, acetylene + ammonia, acetylidene, alcohols, aldehydes, alkalis, alkali hydroxides, ammonia, ammonium compounds, antimony salts, arsenites, benzalkonium chloride, bromides, carbonates, carbides, charcoal, chlorides, chlorosulfonic acid, creosote, ferrous salts, halogenated acids and their salts, hydrazine and derivatives, hydrogen peroxide, hydrogen sulfide, hypophosphites, iodides, magnesium in powder form (with water), morphine salts, nitriles, non-metals, oils, organic nitro compounds, phosphates, sodium hydroxide, sugars, tannic acid, tannins, tartrates, thimerosal, thiocyanates, vegetable decoctions, and extracts, volatile oils.
Hazardous Decomposition Products: Oxygen, toxic fumes, nitrous gases, toxic oxides of nitrogen, silver/silver oxides.
Hazardous Decomposition Products: Oxygen, toxic fumes, nitrous gases, toxic oxides of nitrogen, silver/silver oxides.
11. TOXICOLOGICAL INFORMATION
Acute effects
No further relevant information available.
Eye contact
Corrosive. Can cause blurred vision, redness, pain, severe tissue burns and eye damage.
Skin contact
Corrosive. Symptoms of redness, pain, and severe burn can occur.
Ingestion
Corrosive. Swallowing can cause severe burns of the mouth, throat, stomach and gastrointestinal tract.Can cause sore throat, vomiting, diarrhoea. Poison. Symptoms include pain and burning in the mouth, blackening of the skin and mucous membranes, throat, and abdomen, salivation, vomiting of black material, diarrhoea, collapse, shock, coma and death. Ingestion of soluble silver salts may cause argyria, characterised by permanent blue-gray pigmentation of the skin, mucous membranes, and eyes. Lethal dose for humans is 2 grams, or about 28.6 mg/kg.
Inhalation
Extremely destructive to tissues of the mucous membranes and upper respiratory tract. Symptoms may include severe irritation, burning sensation, coughing, wheezing, laryngitis, shortness of breath, breathing difficulty, headache, nausea, vomiting and possible coma. May be absorbed into the body following inhalation with symptoms paralleling those from ingestion exposure. Dust deposits in the lungs may resemble a form of pneumoconiosis. Inhalation of silver metal dust and fume or of soluble silver compounds may eventually cause argyria, an unsightly blue-gray discoloration of the skin and mucous membranes, including gum tissue and conjunctiva of the eyes.
Toxicity and irritation
* Nitrate or nitrite (ingested) under conditions that result in endogenous nitrosation are evaluated in the IARC Monographs (Vol. 94; in preparation) as Group 2A: Probably carcinogenic to humans.* Carcinogenicity Evidence of reproductive effects. May cause methemoglobinemia, which is characterised by chocolate-brown coloured blood, headache, weakness, dizziness, breath shortness, cyanosis (bluish skin due to deficient oxygenation of blood), rapid heart rate, unconsciousness and possible death. Repeated inhalation may cause lung disease. Chronic inhalation or ingestion of silver salts may cause argyria characterised by a permanent blue-gray discolouration of the eyes, skin, mucous membranes, and internal organs. This malady results from the accumulation of silver in the body. Laboratory experiments have shown mutagenic effects.
12. ECOLOGICAL INFORMATION
Ecotoxicity
Highly toxic for aquatic organisms. May cause long-term adverse effects in the aquatic environment. Forms corrosive mixtures with water even if diluted.
Persistence and degradability
Methods for the determination of biodegradability are not applicable to inorganic substances.
Bioaccumulative potential
BCF: 200; Highly bioaccumulative (Biological Concentration Factor 100-1000). Do not allow to enter waters, waste water, or soil!
Other adverse effects
No further relevant information available.
13. DISPOSAL CONSIDERATIONS
General information
Dispose of according to relevant local, state and federal government regulations.
14. TRANSPORT INFORMATION
ADG label required
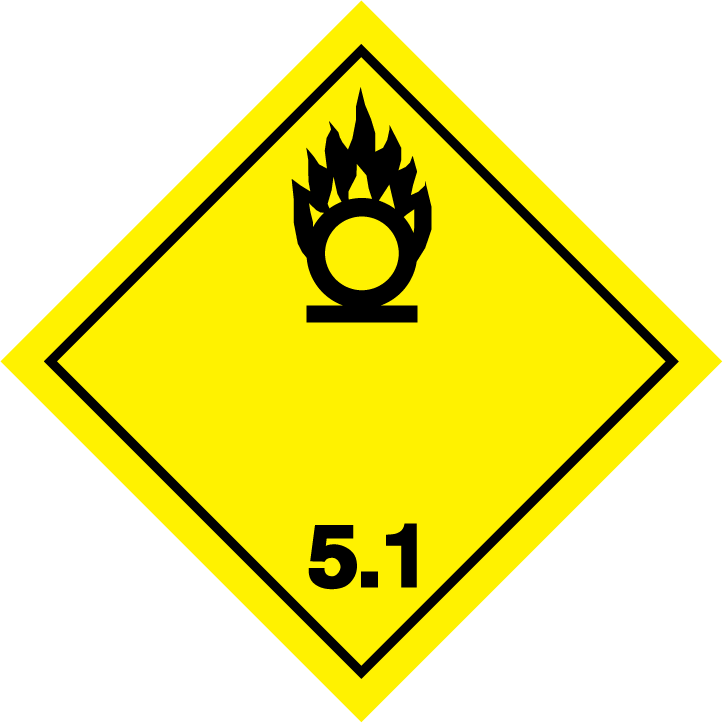
HAZCHEM
1Y
UN Number
UN1493
Proper shipping name
SILVER NITRATE
Transport hazard class
5.1
Packing group
PG II
Environmental hazard
No information available
Special precautions for users
No information available
Additional information
Packaging Method 3.8.5.1
EPG Number 5B1
IERG Number 31
EPG Number 5B1
IERG Number 31
15. REGULATORY INFORMATION
Poisons Schedule Number
S6
Other Information
Listed in the Australian Inventory of Chemical Substances (AICS)
16. OTHER INFORMATION
SDS preparation date
8 December 2021
Comments
This information is based on our present knowledge. However, this shall not constitute a guarantee for any specific product features and shall not establish a legally valid contractual relationship.
References
'Standard for the Uniform Scheduling of Medicines and Poisons No. 6', Commonwealth of Australia, February 2015.
Lewis, Richard J. Sr. 'Hawley's Condensed Chemical Dictionary 13th. Ed.', Rev., John Wiley and Sons, Inc., NY, 1997.
National Road Transport Commission, 'Australian Code for the Transport of Dangerous Goods by Road and Rail 7th. Ed.', 2007.
Safe Work Australia, 'National Code of Practice for the Preparation of Safety Data Sheets for Hazardous Chemicals', 2011.
Standards Australia, 'SAA/SNZ HB 76:2010 Dangerous Goods - Initial Emergency Response Guide', Standards Australia/Standards New Zealand, 2010.
Safe Work Australia, 'Approved Criteria for Classifying Hazardous Substances [NOHSC:1008 (2004)]'.
Safe Work Australia, 'Hazardous Substances Information System, 2005'.
Safe Work Australia, 'National Code of Practice for the Labelling of Safe Work Hazardous Substances (2011)'.
Safe Work Australia, 'National Exposure Standards for Atmospheric Contaminants in the Occupational Environment [NOHSC:1003(1995) 3rd Edition]'.
Lewis, Richard J. Sr. 'Hawley's Condensed Chemical Dictionary 13th. Ed.', Rev., John Wiley and Sons, Inc., NY, 1997.
National Road Transport Commission, 'Australian Code for the Transport of Dangerous Goods by Road and Rail 7th. Ed.', 2007.
Safe Work Australia, 'National Code of Practice for the Preparation of Safety Data Sheets for Hazardous Chemicals', 2011.
Standards Australia, 'SAA/SNZ HB 76:2010 Dangerous Goods - Initial Emergency Response Guide', Standards Australia/Standards New Zealand, 2010.
Safe Work Australia, 'Approved Criteria for Classifying Hazardous Substances [NOHSC:1008 (2004)]'.
Safe Work Australia, 'Hazardous Substances Information System, 2005'.
Safe Work Australia, 'National Code of Practice for the Labelling of Safe Work Hazardous Substances (2011)'.
Safe Work Australia, 'National Exposure Standards for Atmospheric Contaminants in the Occupational Environment [NOHSC:1003(1995) 3rd Edition]'.
This Safety Data Sheet (SDS) has been prepared in compliance with the Preparation of Safety Data Sheets for Hazardous Chemicals Code of Practice February 2016. It is the user's responsibility to determine the suitability of this information for adoption of necessary safety precautions. The information published in this SDS has been compiled from the publications listed in Section 16: to the best of our ability and knowledge these publications are considered accurate. We reserve the right to revise Safety Data Sheets as new information becomes available. Copies may be made for non-profit use.